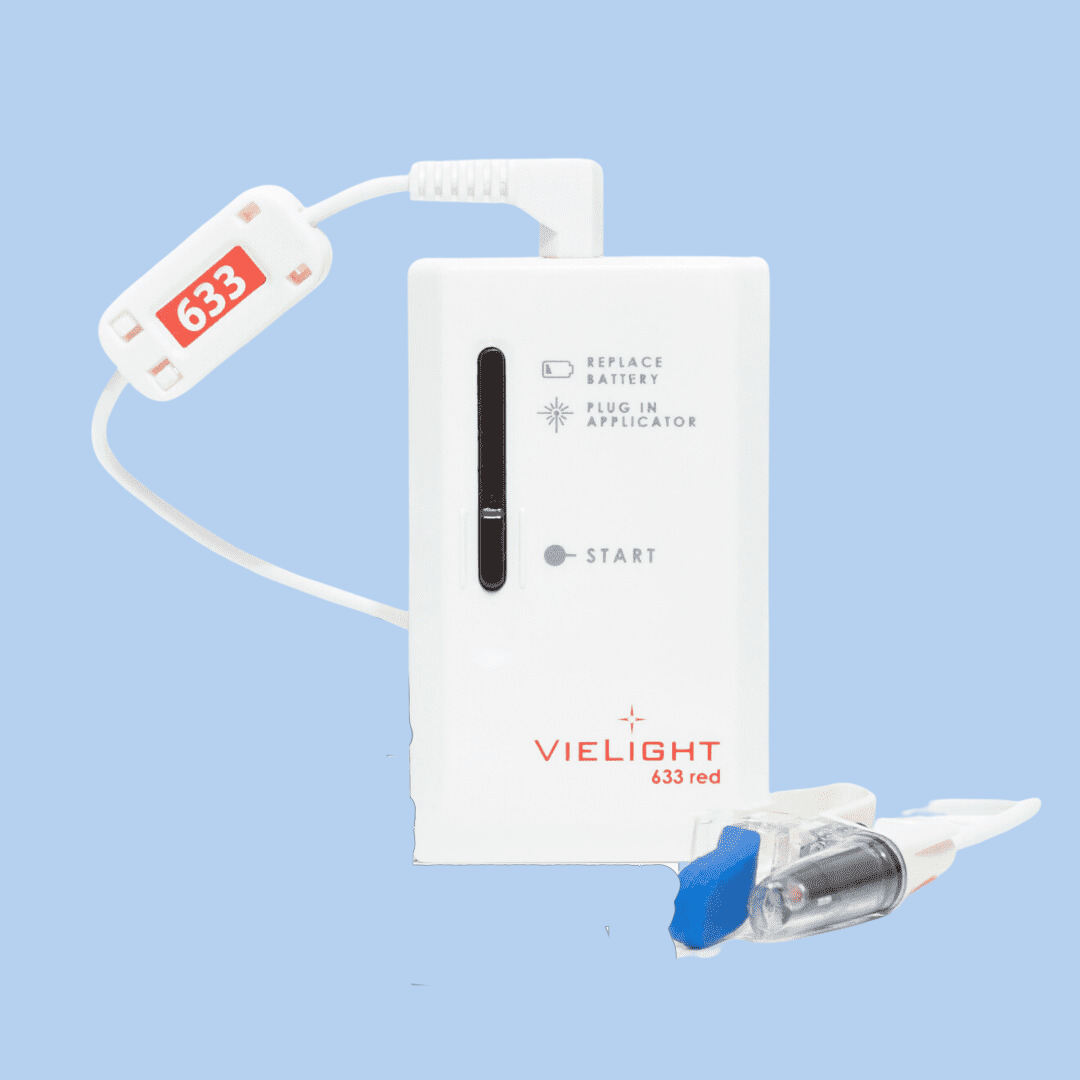
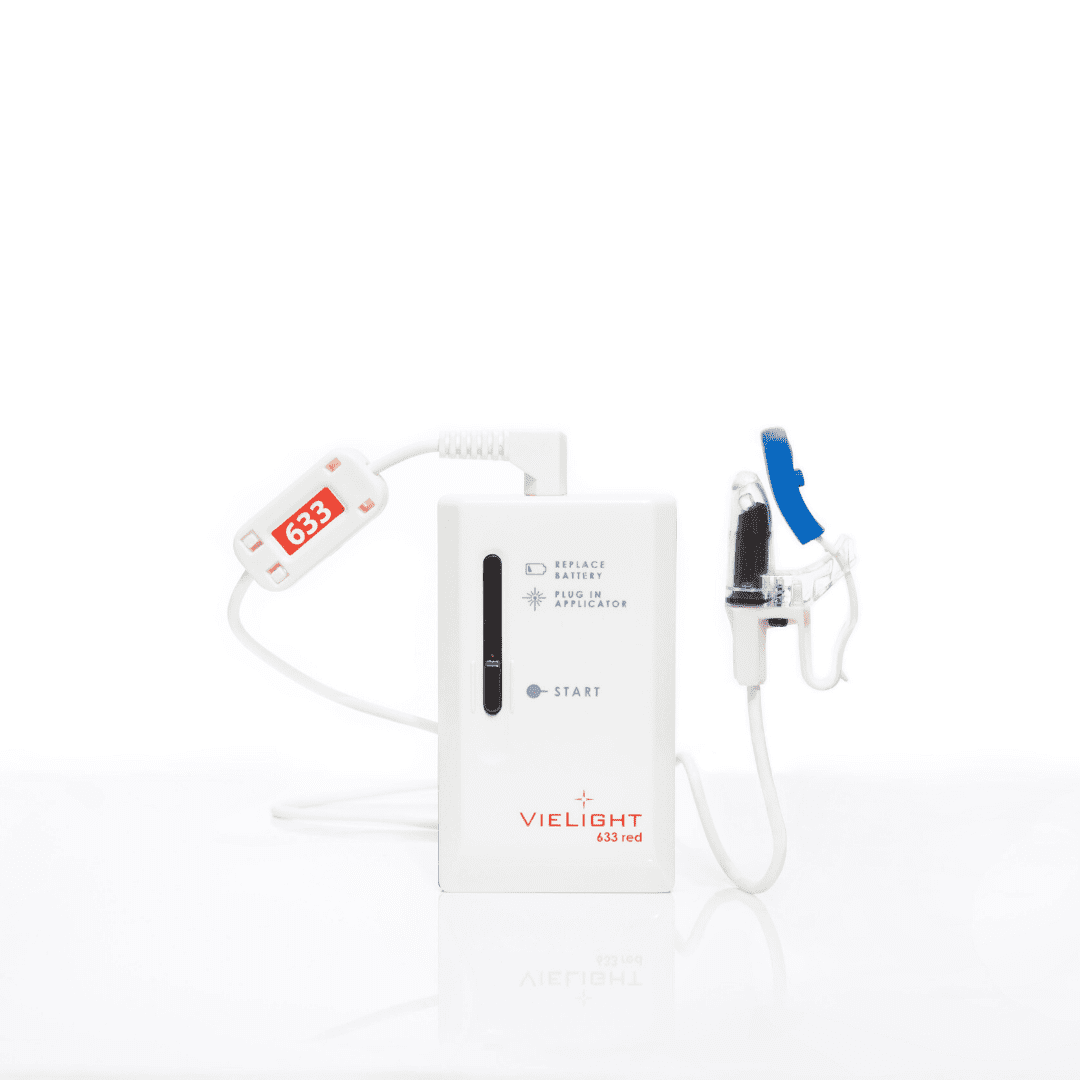
Description
Why intranasal?
The nasal cavity is rich with blood capillaries. There are more capillaries per cm2 than in most organs of the body. Five arteries connect to the nasal cavity, while the Little’s area is a frequent site of nasal bleeding. Given the rich supply of blood and the thin, permeable membrane within the nasal cavity, the light can easily reach the blood. Thus, this area is well-suited for systemic photbiomodulation which via blood.
What is systemic photobiomodulation? : (Link)
The science behind the difference between the 655 nm and 810 nm wavelengths can be found in this independent study : (Link)
Warranty: One-year manufacturer’s warranty
Manual: Link
Returns: Your purchases are backed by a 6-months no-question-asked-money-back guarantee (less 20% of retail price).
Our customer support, satisfaction guarantee and product warranty will not apply to devices sold through unauthorized channels, such as Amazon and eBay.
More Info: Please note that this product comes with an intranasal applicator





Reviews
There are no reviews yet.